Biotech start-ups face unique challenges in securing funding, often requiring innovative approaches to overcome financial hurdles. The journey from concept to commercialization demands significant investment, making it crucial for entrepreneurs to understand the intricacies of growth funding. Whether it's early-stage trials or scaling operations, the right strategies can make all the difference.
Your discussion of growth funding methods is enriched by the detailed perspective provided in biotech startup fundraising strategies, which lays the foundation for a comprehensive funding framework. This guide explores the essential tools and tactics that empower biotech ventures to thrive in a competitive landscape. Let's jump right in!
Strategic Funding Approaches for Biotech: Overcoming the Data-Capital Paradox
Biotech companies often face a unique challenge: the need to generate robust data to secure funding while requiring capital to produce that very data. This paradox has driven the industry to adopt innovative funding strategies that balance early-stage financing with proof-of-concept validation.
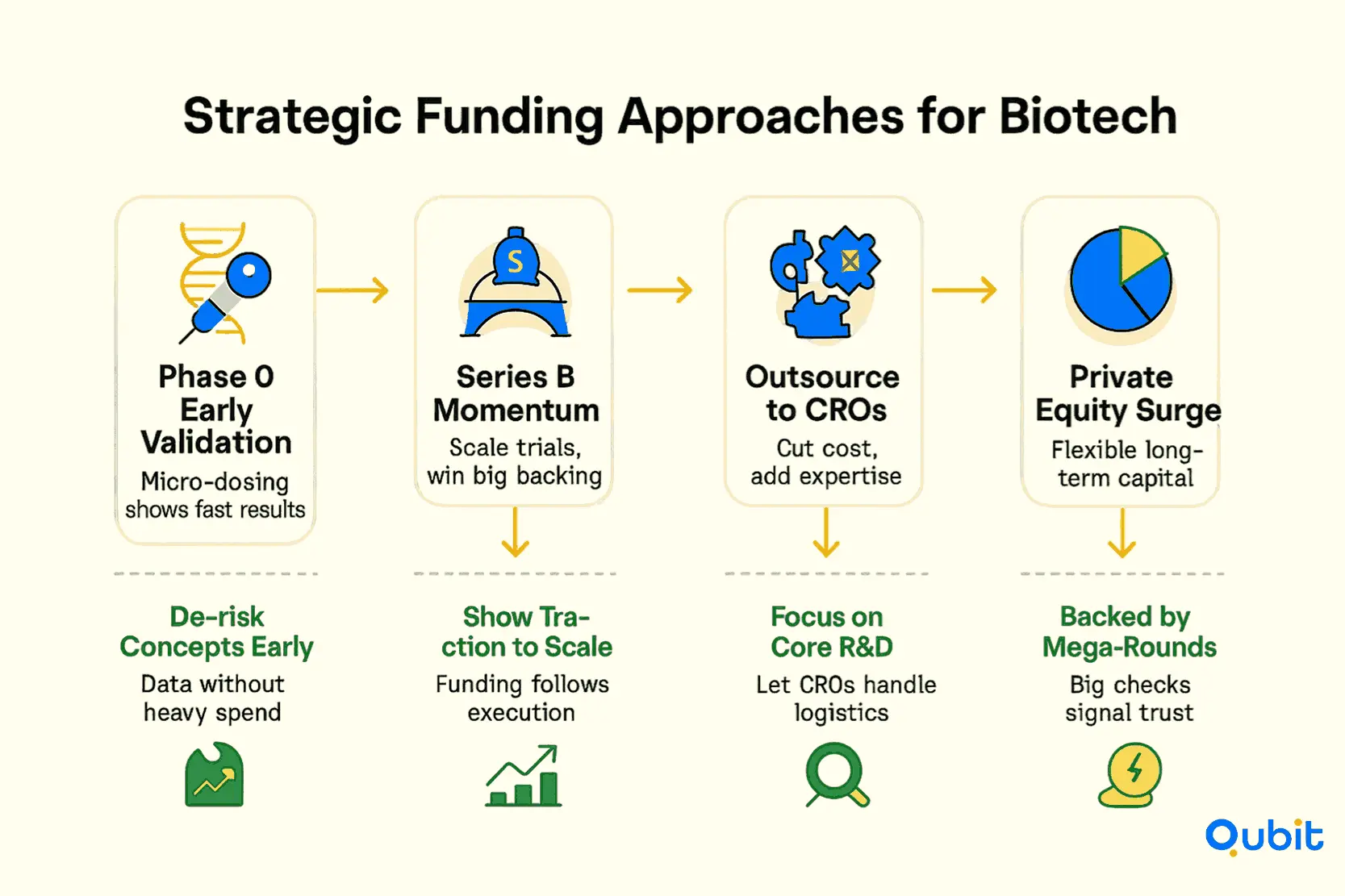
1. Early Validation Through Phase 0 Trials
Phase 0 trials have emerged as a critical tool for biotech companies aiming to de-risk their projects early in development. These trials focus on generating preliminary human data, often with micro-dosing, to validate a concept before committing to larger, more expensive studies. This approach not only accelerates the timeline for proof-of-concept but also provides investors with tangible evidence of a project’s viability.
For example, Phase 0 trials can demonstrate the pharmacokinetics of a drug candidate, offering insights into its potential efficacy and safety. By presenting this data early, biotech firms can attract funding for subsequent phases with reduced risk.
This strategy is particularly effective in addressing the data-capital paradox, as it allows companies to produce meaningful results without requiring substantial upfront investment.
2. Series B Funding Trends: A Window of Opportunity
Series B funding rounds have become a pivotal stage for biotech companies seeking to scale their operations. According to the FierceBiotech Fundraising Tracker (2024), Series B and D rounds continue to show strong investor confidence, even in a leaner market environment. These rounds often focus on expanding clinical trials, scaling manufacturing capabilities, and preparing for commercialization.
One standout example is Xaira Therapeutics, which successfully transitioned from Series A to Series B fundraising while integrating innovative R&D approaches. Their ability to secure significant funding highlights the importance of demonstrating progress and scalability to investors.
3. Strategic Outsourcing to Optimize Capital Allocation
Strategic outsourcing has become a cornerstone for biotech firms looking to minimize overhead while maximizing output. By partnering with Contract Research Organizations (CROs), companies can access specialized expertise and infrastructure without the need for substantial upfront investments. This approach not only reduces operational costs but also enhances the credibility of a project by leveraging established industry players.
For instance, outsourcing preclinical studies or manufacturing tasks allows biotech firms to focus their resources on core R&D activities, ensuring efficient capital allocation.
4. Private Equity’s Growing Role
Private equity is playing an increasingly significant role in shaping strategic partnerships within the biotech sector. With over 50% of funding now coming from private equity sources, companies have more opportunities to secure flexible financing tailored to their specific needs. This trend underscores the importance of aligning funding strategies with long-term goals, whether through equity investments or strategic alliances.
The dominance of mega-rounds further signals investor confidence, even in challenging market conditions. ArsenalBio’s large Series C funding round serves as a prime example of how private equity can drive advanced therapy development and commercialization efforts.
Industry Growth and Early-Stage Investments
The biotech industry raised a total of $26 billion in 2024, with 207 seed and Series A rounds accounting for $9.4 billion. These figures highlight the scale of early-stage investments and the growing appetite for innovation in the sector.
By combining early validation strategies like Phase 0 trials with strategic outsourcing and private equity partnerships, biotech firms can overcome the data-capital paradox and secure the funding needed to advance their projects.
Innovating for Growth: Maximizing R&D Investment in Biotech
Biotech companies are redefining the boundaries of science, and at the heart of this transformation lies robust investment in research and development (R&D). With biopharma R&D spending projected to grow by 11% year-over-year, this surge is fueling advancements in immunology, oncology, and gene therapies.
However, the challenge lies not only in increasing budgets but also in managing these expenditures effectively to maintain market competitiveness and drive innovation.
Alternative Financing Options for Sustained Growth
While traditional venture rounds remain a popular choice for funding, alternative financing methods are gaining traction. Options like venture debt and royalty financing provide biotech firms with the flexibility to sustain R&D efforts without diluting equity. The analysis of alternative capital sources is complemented by the examination found in biotech venture debt royalty financing, which outlines financing options beyond traditional equity.
These methods not only support ongoing innovation but also enable companies to maintain operational stability during periods of high expenditure. By diversifying funding sources, biotech firms can ensure that their R&D pipelines remain active and competitive.
Driving Breakthroughs in Key Areas
The increasing R&D budgets are translating into tangible advancements in critical fields. Immunology research is benefiting from enhanced funding, leading to novel treatments for autoimmune diseases. Similarly, oncology is witnessing the development of targeted therapies that promise improved patient outcomes. Gene therapies, a rapidly evolving domain, are unlocking possibilities for curing genetic disorders that were previously deemed untreatable.
These breakthroughs underscore the importance of strategic R&D investment. By focusing on areas with the highest potential for innovation, biotech companies can not only address unmet medical needs but also secure their position as leaders in the industry.
Achieving Financial & Operational Excellence for IPO Readiness
Scaling operations effectively while preparing for an IPO demands a meticulous approach to financial and operational systems. Robust financial tools, automation, and advanced planning capabilities are essential to ensure compliance, agility, and scalability during this transformative phase.
Building Robust Financial Systems
A solid financial foundation is critical for IPO readiness. Next-generation ERP solutions, such as NetSuite, play a pivotal role in managing sophisticated financial reporting and ensuring compliance with regulatory requirements. Cloud-based ERP systems further accelerate due diligence processes, enabling organizations to meet stringent IPO timelines.
Companies like Viracta Therapeutics exemplify the importance of developing in-house expertise prior to going public. Their strategic focus on financial evolution highlights the value of integrating advanced tools early in the process.
For a deeper understanding of the precursor stages that underpin public market entries, explore the insights provided in biotech IPO SPAC preparation.
Automating Operational Processes
Operational inefficiencies can hinder growth during IPO preparation. Automation tools such as Prendio and Coupa streamline accounts payable (AP) processes, improving invoice conversion rates and procurement integration. For expense reporting, solutions like Concur ensure compliance with regulations such as the Sunshine Act, while Expensify offers simpler management in less regulated environments.
By addressing these inefficiencies, businesses can maintain agility and compliance, even during rapid expansion.
Advanced FP&A Tools for Strategic Planning
Financial planning and analysis (FP&A) tools are indispensable for scenario planning and multi-version budgeting. Adaptive and Planful are two leading platforms that support complex financial modeling, enabling organizations to anticipate challenges and optimize resource allocation.
Multi-version budgeting, in particular, ensures flexibility and accuracy in financial planning, which is crucial for navigating the unpredictable demands of IPO preparation.
Overcoming Integration Challenges
Integrating third-party financial tools with existing systems can be complex, especially in industries like biopharma. Sikich’s insights illustrate how merging finance tools with R&D data systems requires careful planning and execution. Addressing these challenges early can prevent disruptions and ensure seamless operations during IPO readiness.
The Role of Cloud-Based ERP
Adopting cloud-based ERP systems is a game-changer for IPO readiness. These systems enhance compliance, streamline reporting, and provide real-time data access, all of which are essential for meeting regulatory and investor expectations.
By prioritizing financial and operational excellence, businesses can position themselves for a successful IPO, ensuring scalability and long-term growth.
Enhancing Investor Relations through Targeted Matchmaking
Securing biotech funding requires more than just a compelling idea—it demands a strategic approach to investor relations. By understanding investor profiles, crafting tailored pitches, and employing precise matchmaking techniques, biotech startups can optimize funding outcomes while effectively managing dilution.
Understanding Investor Profiles
Every investor has unique priorities, whether they focus on early-stage innovation, long-term growth, or risk mitigation. Segmenting investors into clear categories allows startups to align their funding strategies with the expectations of each group. For instance, some investors may prioritize groundbreaking research, while others may seek scalable business models with proven market traction.
A targeted approach ensures that startups present their value proposition in a way that resonates with each investor segment. The Investor Matchmaking Trend highlights the importance of building customized pitch decks for each category, ensuring that the narrative aligns with the investor's specific interests and goals.
Crafting Tailored Pitches
Generic pitches often fail to capture the attention of discerning investors. Instead, biotech startups should focus on creating personalized presentations that address the unique concerns and aspirations of each investor. This involves:
- Highlighting relevant expertise: Showcase how your team’s background aligns with the investor’s focus area.
- Demonstrating market potential: Provide data-driven insights into the scalability and profitability of your biotech solution.
- Addressing risks upfront: Anticipate potential challenges and present clear strategies to mitigate them.
By tailoring pitches to specific investor profiles, startups can foster stronger connections and increase the likelihood of securing funding. If your data room is growing faster than your finance process, biotech cfo vs fractional support compares responsibilities across forecasting, vendor risk, and compliance.
Strategic Matchmaking for Funding Success
Investor matchmaking is not just about finding capital—it’s about building partnerships that align with your startup’s vision. Programs like the Regional Initiative for Biotech Start-up Funding exemplify how localized matchmaking approaches can connect startups with investors who understand the nuances of their ecosystem. This initiative focuses on pairing biotech startups with investors who are deeply familiar with regional market dynamics, creating mutually beneficial relationships.
Moreover, effective matchmaking plays a critical role in managing dilution. Strategic approaches to maintaining equity are clarified through the discussion in biotech follow-on round dilution management, which addresses ownership considerations in later funding cycles. By securing follow-on rounds and syndicates, startups can preserve ownership while accessing the capital needed for growth.
Balancing Funding and Ownership
While securing funding is essential, startups must also consider the long-term implications of dilution. Partnering with investors who support follow-on rounds and syndicates can help maintain equity while ensuring sustainable growth. This balance is crucial for startups aiming to scale without compromising their vision or control.
By combining clear investor segmentation, tailored pitches, and strategic matchmaking, biotech startups can not only secure funding but also build lasting relationships that drive innovation and growth.
Conclusion
Scaling biotech start-ups requires a thoughtful blend of strategies that address both operational and financial challenges. Innovative funding models, robust operational readiness, and effective investor matchmaking are essential components of this process. By integrating early de-risking measures, such as Phase 0 trials, with advanced financial and regional initiatives, start-ups can position themselves for sustainable growth.
Combining these approaches not only mitigates risks but also creates a strong foundation for scaling in competitive markets. At Qubit Capital, we understand the unique challenges biotech start-ups face and are committed to helping you secure the funding necessary for growth.
If you’re looking to scale smart across ops and capital, at Qubit we understand Phase 0 prove-outs, CMC/QA readiness, and investor matchmaking. Turn de-risking into momentum with our biotech fundraising assistance and plan your next-stage raise.
Key Takeaways
- Innovative funding strategies, such as Phase 0 trials, help overcome the data versus capital paradox.
- Advanced financial readiness—including robust ERP and FP&A systems—is critical to scaling biotech operations.
- Targeted investor matchmaking and tailored pitch decks boost funding success.
- Regional initiatives provide essential, localized support for early-stage biotech start-ups.
- A comprehensive approach that integrates funding, R&D, and investor relations is key to sustainable growth
Frequently asked Questions
What are best funding options for Biotech?
Biotech startups can secure funding through a combination of early-stage financing methods, such as angel investors and venture capital. Innovative approaches like Phase 0 trials and strategic outsourcing also play a pivotal role in obtaining essential resources for growth.






