Securing funding is a pivotal milestone for any biotech start-up, but attracting investors requires more than just a groundbreaking idea. To stand out in a competitive market, founders must demonstrate not only the potential of their innovation but also the readiness of their business for investment. From organizing essential documents to crafting a compelling pitch, preparation is key to building investor confidence.
Your exploration of biotech financing gains broader context through the insights presented in the biotech startup fundraising strategies, which outlines overarching approaches that set the stage for more detailed discussions.
This article will guide you through the critical steps of investment preparation, including corporate documentation, realistic valuation, investor research, and pitch deck development. Let’s jump right in!
Organize Corporate Documentation and Prepare Data Room
Efficient organization of corporate documentation is essential for biotech companies aiming to attract investors and streamline due diligence processes. A well-structured approach ensures that critical information is readily accessible, fostering transparency and trust during investment evaluations.
Centralize Key Documents in a Digital Repository
Biotech firms often deal with complex documentation, including formation papers, operational agreements, and contracts. To simplify access and enhance security, setting up an Electronic Data Room is highly recommended. This digital repository serves as a centralized hub where all essential corporate documents are stored, categorized, and easily retrievable.
Essential Components of a Data Room
A well-organized data room for biotech should include:
Formation Documents: Articles of incorporation, bylaws, and shareholder agreements.
Financial Records: Audited financial statements, tax filings, and projections.
Contracts: Licensing agreements, vendor contracts, and partnership details.
Intellectual Property: Patents, trademarks, and proprietary research data.
Operational Records: Employee agreements, compliance certifications, and regulatory filings.
Each document should be clearly labeled and categorized to avoid confusion and delays during the review process.
Biotech Startup's Investment Readiness
A well-crafted business plan, combined with a skilled team and validated technology, can significantly elevate your biotech startup’s appeal to potential investors.
The Power of a Comprehensive Business Plan
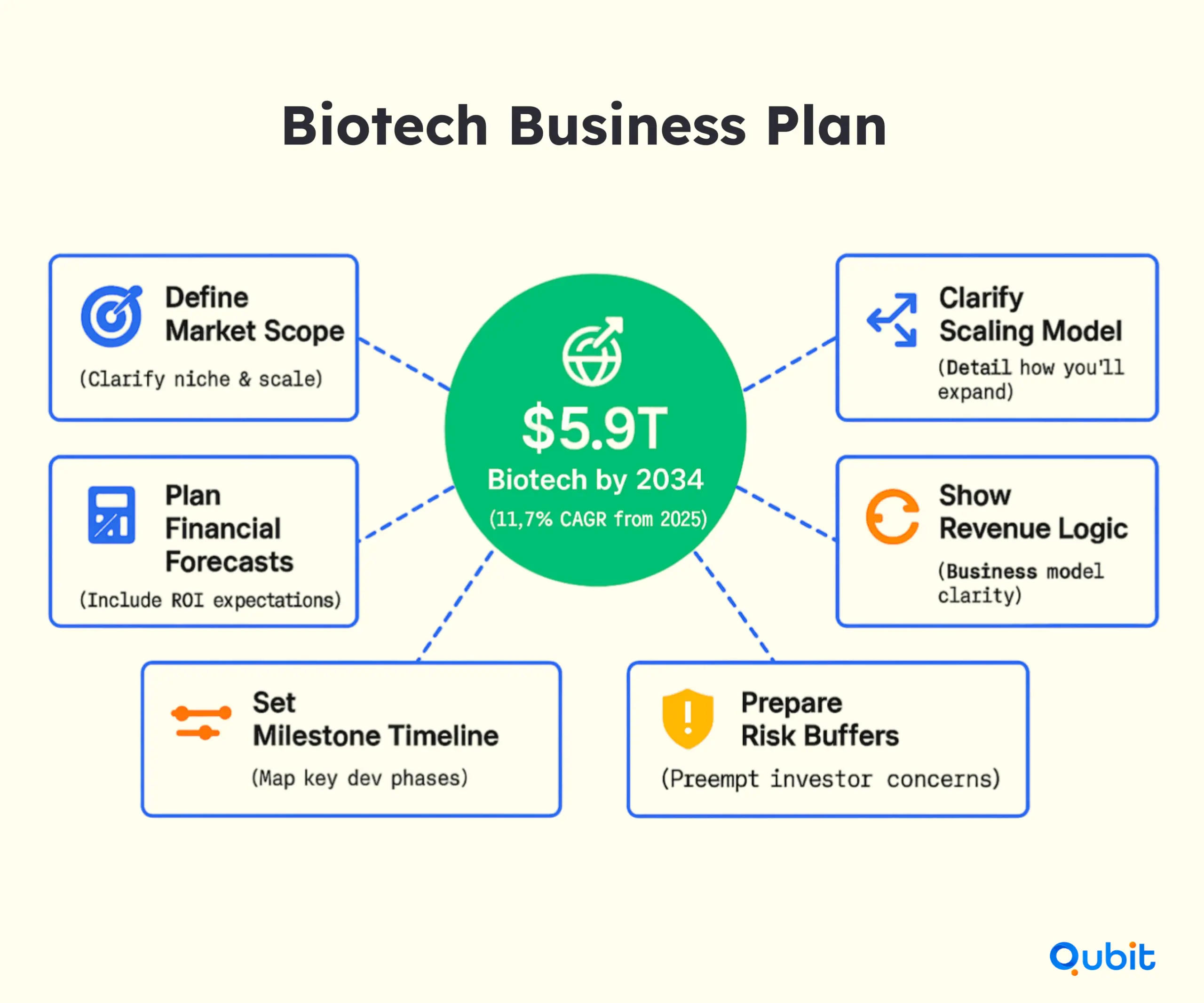
A strong business plan acts as the backbone of your investment strategy. It should clearly articulate your market opportunity, financial projections, and development milestones. For biotech startups, this is especially critical given the sector’s complexity. Highlighting the massive potential of the industry, the Global Biotech Market Size is projected to reach $5.90 trillion by 2034, growing at an impressive 11.7% CAGR from 2025. This underscores the vast opportunities available for startups to tap into.
Investors want to see a roadmap that demonstrates not only your vision but also your ability to execute it. Tools like a Pitch Deck / Business Plan can help you present your startup as a compelling investment opportunity. By detailing your strategy for scaling, revenue generation, and risk mitigation, you can build confidence in your ability to deliver results.
Team Expertise: A Critical Factor
The strength of your team is often a deciding factor for investors. Biotech startups require a multidisciplinary approach, blending scientific expertise with business acumen. Investors look for teams that can navigate the complexities of regulatory approvals, clinical trials, and commercialization.
For example, Moderna Therapeutics showcased the importance of team expertise during its platform expansion financing. In 2015, the company raised $450 million to scale its mRNA therapeutics across multiple therapeutic areas. This funding accelerated pipeline development and positioned Moderna as a leader in mRNA vaccines. Such success stories highlight how a capable team can drive innovation and attract significant investment.
Validating Your Technology
Scientific validation is non-negotiable in biotech. Investors need assurance that your technology is both feasible and scalable. Conducting feasibility studies, securing patents, and publishing peer-reviewed research can bolster your credibility.
Additionally, safeguarding intellectual property is essential to protect your competitive edge. Investors are more likely to back startups that demonstrate a clear strategy for maintaining exclusivity in their innovations.
For further insights into investor expectations, explore what do biotech investors want, which delves into the criteria influencing investment decisions in the biotech sector.
Realistic Pre-Money Valuation and Funding Milestones
Determining a realistic pre-money valuation biotech is crucial for startups aiming to secure funding while maintaining credibility with investors. This valuation reflects the company’s worth before external financing and must align with market trends and the developmental stage of the business.
The Role of Market Trends in Valuation
Investors are increasingly drawn to biotech ventures, as evidenced by the Median Biotech Venture Round reaching $93M in Q1 2025. This figure, sourced from BioPharma Dive, highlights a consistent trend of larger financings since 2024. Incorporating such data into valuation discussions demonstrates the growing investor interest in the sector, providing startups with a benchmark to assess their own worth.
Aligning Funding with Milestones
Biotech startups must align funding amounts with key funding milestones biotech, such as clinical trials, regulatory approvals, or product launches. This approach ensures capital is directed toward achieving measurable progress, reducing the risk of overspending or underfunding critical phases.
Balancing Ambition and Realism
While ambitious valuations can attract attention, they may also deter cautious investors. Striking a balance between ambition and realism is essential. A valuation that reflects both the company’s potential and its current achievements fosters trust and sets the stage for long-term partnerships.
Understanding these dynamics not only helps startups secure funding but also positions them for sustainable growth in a competitive industry.
Understanding Financing Types and Terms
Biotech startups often face unique challenges when securing funding, requiring a clear understanding of financing types and terms to make informed decisions. Whether exploring priced rounds or unpriced rounds, knowing the distinctions can shape the trajectory of your venture.
Types of Financing for Biotech Startups
Biotech startups typically encounter two primary financing structures: priced rounds and unpriced rounds.
- Priced Rounds: These involve assigning a valuation to the company before investment. Investors purchase equity based on this valuation, providing clarity on ownership stakes. Priced rounds are ideal for startups with established milestones and predictable growth trajectories.
- Unpriced Rounds: Commonly structured as convertible notes or SAFE agreements, unpriced rounds defer valuation discussions. They offer flexibility for early-stage startups still refining their business models or awaiting critical milestones.
Key Components of Term Sheets
Term sheets serve as the foundation for investment agreements, outlining the terms and conditions of funding. For biotech startups, these documents often include:
- Valuation Terms: Establishing the pre-money and post-money valuation.
- Investor Rights: Details on voting rights, board representation, and liquidation preferences.
- Milestone-Based Funding: Specific conditions tied to achieving developmental or regulatory milestones.
Negotiating term sheets requires careful attention to detail, ensuring alignment between investor expectations and the startup’s long-term vision.
The analysis of monetary planning and growth milestones is complemented by the details in financial modelling biotech seed round, which reviews structured financial forecasts alongside developmental targets.
Strategic Considerations
Selecting the right financing type and negotiating favorable term sheets are pivotal steps for biotech startups. A strategic approach ensures that funding supports both immediate needs and long-term growth.
Targeting the Right Investors
39% of biotechs had less than one year of cash runway in 2024, marking the highest level in six years. This statistic underscores the urgency of identifying investors whose strategies align with your company’s stage and vision.
Why Specialized Investors Matter
Investors are increasingly focusing on specific sectors, stages, and investment theses. This trend, known as specialized investors, highlights the importance of targeted investor outreach. Biotech entrepreneurs should prioritize communities that understand their niche and can provide not just capital but also strategic guidance. These investors often have deep expertise in areas like early-stage drug development or clinical trials, making them invaluable partners for startups navigating complex industry demands.
Strategies for Investor Research in Biotech
Define Your Funding Needs
Start by assessing your current cash runway and growth objectives. Understanding whether you need seed funding, Series A, or later-stage investment will help narrow your search to investors who specialize in your stage.Identify Sector-Specific Investors
Research investors who focus exclusively on biotech or life sciences. Their familiarity with regulatory hurdles and scientific innovation can accelerate your fundraising efforts.Tailor Your Outreach
Craft personalized pitches that align with each investor’s thesis. Highlight how your startup’s goals resonate with their portfolio and expertise.
The Investor Lifecycle
Targeting the right investors isn’t just about immediate funding; it’s about ensuring future financing capabilities. Building relationships with specialized investors early can pave the way for follow-on funding rounds, creating a sustainable growth trajectory for your biotech startup.
Exploring the Funding Landscape for Early-Stage Biotech
Securing funding is a critical step for early-stage biotech startups aiming to bring innovative solutions to market. The high costs associated with research, development, and regulatory approval often make this process challenging, but a variety of funding options exist to support these ventures.
Government Grants: A Reliable Starting Point
Government grants provide a solid foundation for biotech startups, particularly those focused on groundbreaking research. Programs like SBIR grants offer financial support to small businesses engaged in scientific innovation. These grants are highly competitive but can be transformative for startups seeking non-dilutive funding to advance their projects.
Crowdfunding Platforms: Engaging the Public
Crowdfunding has emerged as an alternative funding method for biotech companies. Platforms such as Kickstarter and Indiegogo enable startups to raise smaller amounts of capital from individual contributors. This approach not only generates funds but also builds community support and awareness for the company’s mission.
Venture Capital and Megarounds
Biotech venture capital remains a cornerstone for startups requiring substantial investment. The rise of "megarounds," which involve financing amounts exceeding $100 million, highlights the growing scale of investment in the sector. While these rounds are typically reserved for companies with proven potential, they underscore the immense opportunities available for biotech innovators.
Addressing Fundraising Challenges
Emerging biotechnology companies face significant hurdles in securing funding due to the high costs of bringing life science products to market. These challenges often require startups to demonstrate innovation validation and navigate complex regulatory landscapes. Diversifying funding sources and building strong investor relationships can help overcome these obstacles.
Early-stage biotech funding is multifaceted, offering opportunities through government grants, crowdfunding, and venture capital. By exploring these avenues, startups can position themselves for growth while addressing the unique challenges of the industry.
Crafting an Engaging Investor Pitch
For biotech startups, crafting an investor pitch that resonates involves blending innovation with strategic foresight. Here is what you can do:
Highlighting Market Opportunities
Investors are drawn to pitches that clearly define the problem and outline the solution. For instance, Ginkgo Bioworks successfully accessed public capital by merging with Soaring Eagle through a SPAC strategy. This approach not only infused $2.5 billion in capital but also positioned the company at a valuation of $17.8 billion. Highlighting such alternative pathways to public markets can demonstrate adaptability and strategic thinking.
Integrating Trends into Your Pitch
Incorporating industry trends can elevate your pitch. The growing adoption of AI in R&D is a prime example. By showcasing how AI can reduce drug discovery timelines, you align your pitch with cutting-edge advancements that investors find promising. This integration reflects your awareness of industry dynamics and your ability to capitalize on them.
Structuring Your Pitch Deck
A well-structured pitch deck is essential for capturing investor attention. To refine your presentation strategies, explore the practical insights offered in the biotech pitch deck example. This resource illustrates formats and structures that align with investor expectations, ensuring your pitch is both professional and impactful.
Financial Projections and Exit Strategies
Investors seek clarity in financial forecasts and exit strategies. Presenting robust projections and outlining potential returns can instill confidence in your business model. Whether through traditional IPOs or innovative SPAC mergers, showcasing viable pathways to profitability is crucial for attracting interest.
By combining a clear narrative, market insights, and strategic presentation, your investor pitch can stand out in the competitive biotech landscape.
Establishing strong investor relations in biotech is essential for securing financial support and fostering long-term partnerships. Networking plays a pivotal role in this process, connecting biotech companies with investors who share their vision and goals. By cultivating these relationships, businesses can access the resources needed to advance their innovations and achieve milestones.
Building Investor Relations Through Effective Networking
1. Understanding the Role of Networking in Biotech
Networking is more than just exchanging business cards; it’s about creating meaningful connections that drive mutual success. For biotech companies, this means engaging with investors who understand the complexities of the industry and are willing to support groundbreaking research. Networking events, conferences, and one-on-one meetings provide opportunities to showcase your company’s potential and align with investors’ interests.
2. Case Study: Sionna Therapeutics Series C Financing
A prime example of effective networking in biotech is the success of Sionna Therapeutics. Facing the challenge of advancing its cystic fibrosis pipeline, Sionna secured $182 million in Series C financing with the support of RA Capital and OrbiMed. This funding enabled the company to accelerate its research, with four compounds expected to enter clinical trials by the end of 2024. The ability to connect with private investors who believed in their mission was instrumental in achieving this milestone.
3. Strategies for Strengthening Investor Relations
To build lasting investor relationships, biotech companies can adopt several strategies:
- Set Up Advisory Boards: Establishing advisory boards with industry experts can enhance credibility and attract investor interest.
- Engage in Strategic Partnerships: Collaborating with established organizations can open doors to new funding opportunities.
- Tailor Communication: Investors appreciate transparency and tailored updates that highlight progress and address concerns.
4. Actionable Tips for Networking Success
- Attend Industry Events: Participate in biotech conferences and investor forums to expand your network.
- Prepare a Compelling Pitch: Clearly articulate your company’s vision, milestones, and potential impact.
- Follow Up: Maintain consistent communication with investors to nurture relationships over time.
Effective networking is the cornerstone of building investor relations in biotech. By fostering connections and demonstrating value, companies can secure the support needed to drive innovation forward.
Leveraging Qubit Capital Consulting for Funding Success
Securing funding is often the most critical step for biotech startups aiming to transform groundbreaking ideas into tangible innovations. Qubit Capital's Consulting specializes in guiding biotech innovators through this challenging process, offering tailored strategies to help them thrive. Their expertise in consulting biotech funding ensures startups can access the resources they need to succeed.
Comprehensive Support for Biotech Startups
Qubit Capital Consulting provides a range of services designed to address the unique challenges of biotech funding. From crafting compelling business plans to facilitating investor outreach, their team ensures every aspect of the funding journey is optimized. Additionally, they emphasize intellectual property protection, helping startups safeguard their innovations while attracting investor confidence.
Why Expert Guidance Matters
Funding strategies are not one-size-fits-all. Qubit Capital Consulting understands this and works closely with startups to design approaches tailored to their specific goals and market dynamics. Their deep industry knowledge and strategic insights enable startups to present their value propositions effectively, maximizing their chances of securing investment.
Conclusion
For biotech startups to secure funding it would require a strategic approach that combines preparation, clarity, and relationship-building. By focusing on thorough document organization, setting realistic valuations, crafting a compelling pitch, and fostering strong investor connections, startups can significantly improve their chances of success. These actionable insights highlight the importance of a well-rounded funding strategy tailored to the unique challenges of the biotech industry.
To maximize your potential, consider utilizing expert services that specialize in fundraising strategies. At Qubit Capital, we are committed to helping biotech startups achieve their funding goals. If you're ready to enhance your funding strategy, check out our Fundraising Assistance service to propel your biotech startup forward.
Key Takeaways
- Comprehensive corporate documentation and a secure data room are fundamental for investor due diligence.
- A robust business plan, validated technology, and a strong team are critical for showcasing investment potential.
- Setting a realistic pre-money valuation and aligning funding with milestones optimizes capital usage.
- Understanding diverse financing types and term sheets is essential for negotiating effective deals.
- A compelling investor pitch and strategic networking pave the way for funding success.
Frequently asked Questions
What are the key steps to prepare for biotech startup?
Securing investment begins with a solid foundation. Startups should focus on developing a clear business model, conducting thorough market research, and ensuring regulatory compliance. Additionally, assembling a skilled team and crafting a compelling value proposition are essential steps to attract investors.


 Back
Back



